Redoxmetabolism in Neurons and Cancer
Venkataramani Lab
Role of the Alzheimer's amyloid precursor protein (APP) in iron and redox metabolism
The amyloid precursor protein (APP) plays an essential role in the pathogenesis of Alzheimer's disease. Here, APP is cleaved by beta- and gamma-secretases into A-beta-peptides, which accumulate intra- and extraneuronally in the form of soluble oligomers and solid amyloid plaques. However, under physiological conditions APP is processed via alpha-secretase (ADAM10) that cuts within the A-beta region. The released large soluble fragment sAPP-alpha has synaptotrophic, growth-promoting and cell-protective effects in neuronal and non-neuronal cells (Venkataramani et al., 2012b).
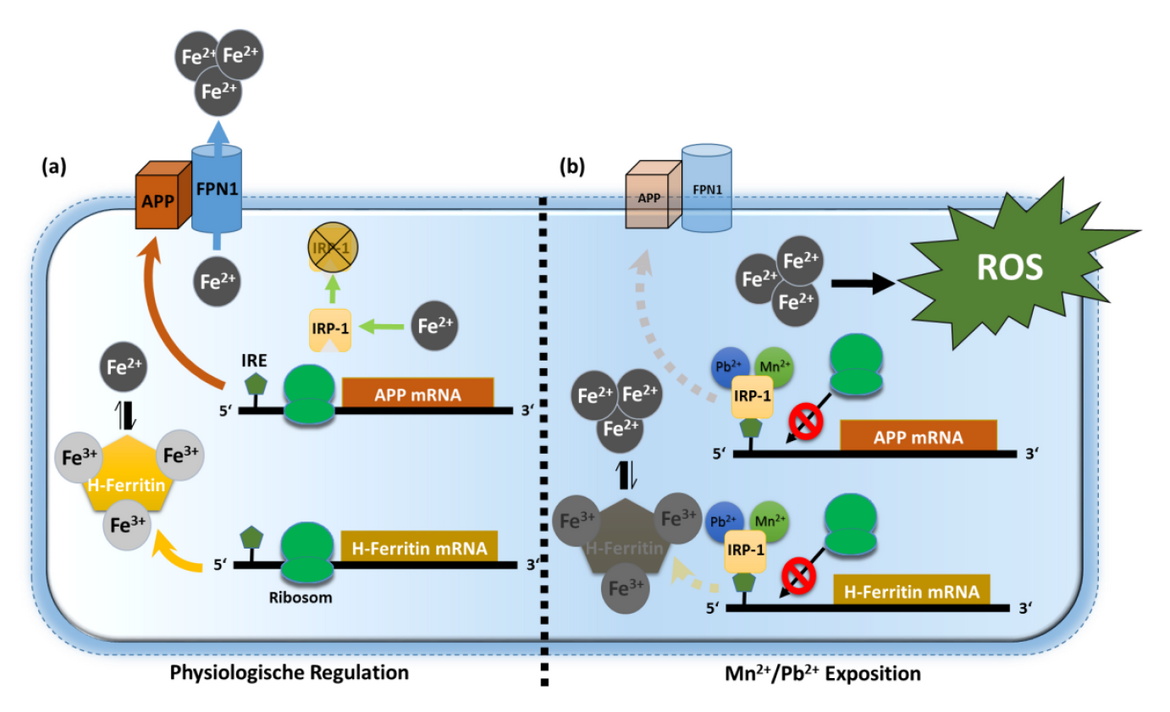
In neuronal cell cultures as well as in various animal models, we found clear evidences that the cellular iron content regulates the expression of APP. In line with other prominent iron regulators, the mRNA of APP contains a functional homologous IRE (iron responsive element)-consensus sequence. The master iron regulator IRP1 can bind to this IRE-like sequence and so block APP protein translation. Moreover, we demonstrated that heavy metals such as lead (Pb2+) and manganese (Mn2+) increase this inhibitory IRE binding and thus block effective protein translation of APP. The loss of APP leads to a dramatic intracellular accumulation of redox-active, divalent iron (Fe2+), which drives lipid peroxidation. Based on our results, we now provide a novel explanation for increased APP protein levels in oxidatively stressed neuronal cells and tissues and how APP shields cells from iron-mediated oxidative stress (Venkataramani et al., 2018a).
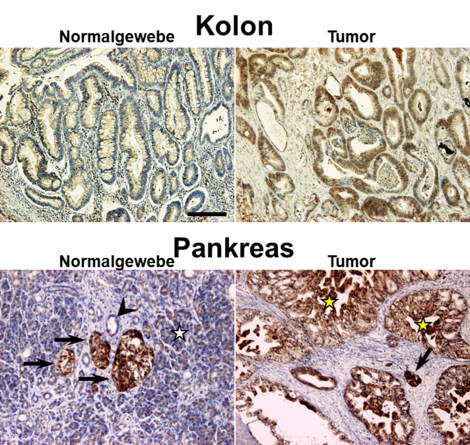
Nearly 10 years ago we first discovered that APP is also highly expressed in various malignant tumor entities including pancreatic, colon and testicular tumors (Venkataramani et al., 2010; Venkataramani et al., 2012a). Interestingly, large amounts of iron are required in the course of tumor development, implicating that high levels of APP protect cancer cells from iron-mediated oxidative stress and cell death.
Indeed, loss of APP in multiple tumor cell models in vitro, ex vivo and in vivo results in significant Fe2+ accumulation, lipid peroxidation and oxidative DNA damage, altogether suppressing tumor growth. These results highlight APP as new druggable target for multiple cancer entities.
CE-ICP-MS: A new method for the quantitative determination of Fe2+ and Fe3+ species in tissues and body fluids
Iron metabolism plays a central role in various physiological processes such as cell growth and division, but also in pathophysiological processes such as neurodegenerative and malignant diseases. The total iron can be divided into a “stable” and “labile” iron pool (LIP). The "stable" iron pool represents thermodynamically stable iron complexes (e.g. metal chaperones), while the "labile" iron pool is the loosely bound, cytoplasmic iron (Fe2+), which can be released easily and readily participates in numerous redox reactions.
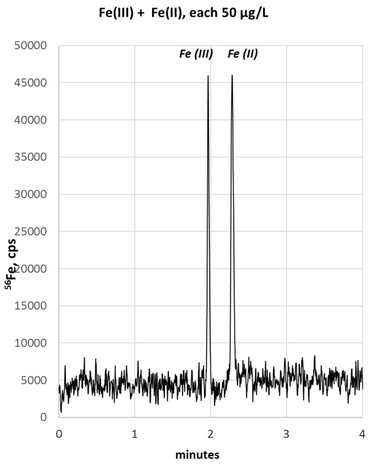
The CE-ICP-MS (Capillary Electrophoresis Coupled to Inductively Coupled Plasma Mass Spectrometry) was developed in close cooperation with Prof. Bernhard Michalke (Helmholtz-Center Munich). With this unique method we now can simultaneously determine absolute amounts of Fe2+ and Fe3+ in preclinical and clinical samples. Compared to the alternative method LC-ICP-MS, our new method offers several advantages with the same reliability. Ongoing studies in biological samples (cell lysates, CSF, tumor samples) will provide evidence whether a quantitative Fe2+/ Fe3+ ratio can be used to reflect Fe2+-mediated tissue damage (biomarker for "tissue at risk") (Michalke et al., 2019; Michalke et al., 2020).
Watch the explanation of the method: https://www.jove.com/v/61055/setup-capillary-electrophoresis-inductively-coupled-plasma-mass
Oxidative stress as a therapeutic strategy for rare soft tissue tumors
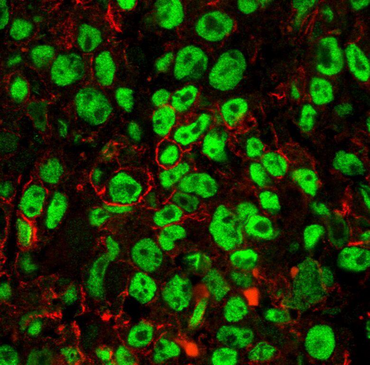
Sarcomas are a broad family of mesenchymal malignancies exhibiting remarkable histologic diversity that altogether account for 1% of adult tumors. These rare tumors arise from the soft tissue, such as muscle, fat or bone and can be classified into more than 170 different types. With around 1 to 2% of all soft tissue sarcomas, angiosarcoma are a very rare, highly malignant sarcoma entity that can either originate from blood vessels (hemangiosarcoma) or lymphatic vessels (lymphangiosarcoma).
In general, the prognosis of angiosarcomas is very poor with 51 months in the localized stage and 12 months in the metastatic stage. Current treatment options are surgical removal, radiation and chemotherapy (such as paclitaxel, doxorubicin and darcabazin). However, incomplete resection of angiosarcomas only moderately or poorly respond to a combined radiochemotherapy and frequently relapse.
The surface marker CD31 is a transmembrane protein that is expressed on endothelial cells, platelets and immune cells. However, the biological function of CD31 in angiosarcoma is still enigmatic. Interestingly, we showed for the first time a heterogeneous CD31 expression in patients and in three established human angiosarcoma cell lines (ASM, ISO-HASc.1 and HAMON). While angiosarcoma cells with high CD31 levels (CD31high) morphologically still resemble normal endothelial cells ("cobblestone" morphology), CD31low cells present a "spindle-shaped" fibroblast-like morphology. Further characterization revealed that the CD31low subpopulation is not only more tumorigenic, but also refractory to conventional chemotherapy.
At the molecular level, we were able to demonstrate that the loss of CD31 prevent the degradation of the Hippopathway regulator YAP (yes-associated protein). In addition to the oncogenic function of YAP, it could be further demonstrated that YAP induces a range of anti-oxidative target genes such as catalase and thioredoxin. These potent antioxidants effectively eliminate toxic radicals and thus reduce oxidative stress levels. Based on this evidence, YAP represents a possible molecular target in aggressive CD31low cells (Venkataramani et al., 2018b).
Selected Publications
- Michalke, B., Willkommen, D., and Venkataramani, V. (2019). Iron Redox Speciation Analysis Using Capillary Electrophoresis Coupled to Inductively Coupled Plasma Mass Spectrometry (CE-ICP-MS). Frontiers in Chemistry 7.
- Michalke, B., Willkommen, D., and Venkataramani, V. (2020). Setup of Capillary Electrophoresis-Inductively Coupled Plasma Mass Spectrometry (CE-ICP-MS) for Quantification of Iron Redox Species (Fe(II), Fe(III)). J Vis Exp.
- Venkataramani, V., Doeppner, T.R., Willkommen, D., Cahill, C.M., Xin, Y., Ye, G., Liu, Y., Southon, A., Aron, A., Au-Yeung, H.Y., et al. (2018a). Manganese causes neurotoxic iron accumulation via translational repression of amyloid precursor protein and H-Ferritin. J Neurochem 147, 831-848.
- Venkataramani, V., Küffer, S., Cheung, K.C.P., Jiang, X., Trümper, L., Wulf, G.G., and Ströbel, P. (2018b). CD31 Expression Determines Redox Status and Chemoresistance in Human Angiosarcomas. Clin Cancer Res 24, 460-473.
- Venkataramani, V., Rossner, C., Iffland, L., Schweyer, S., Tamboli, I.Y., Walter, J., Wirths, O., and Bayer, T.A. (2010). Histone deacetylase inhibitor valproic acid inhibits cancer cell proliferation via down-regulation of the alzheimer amyloid precursor protein. J Biol Chem 285, 10678-10689.
- Venkataramani, V., Thiele, K., Behnes, C.L., Wulf, G.G., Thelen, P., Opitz, L., Salinas-Riester, G., Wirths, O., Bayer, T.A., and Schweyer, S. (2012a). Amyloid Precursor Protein Is a Biomarker for Transformed Human Pluripotent Stem Cells. Am J Pathol.
- Venkataramani, V., Wirths, O., Budka, H., Hartig, W., Kovacs, G.G., and Bayer, T.A. (2012b). Antibody 9D5 recognizes oligomeric pyroglutamate amyloid-beta in a fraction of amyloid-beta deposits in Alzheimer's disease without cross-reactivity with other protein aggregates. J Alzheimers Dis 29, 361-371.
Complete Lists
https://scholar.google.com/citations?user=TBT4ZR0AAAAJ&hl=en
https://www.researchgate.net/profile/Vivek_Venkataramani/publications
https://pubmed.ncbi.nlm.nih.gov/?term=Venkataramani%20Vivek&sort=date
Press releases on our research
Alzheimer/Cancer
- https://www.hna.de/lokales/goettingen/neuer-ansatz-krebstherapie-2672917.html
- https://www.aerzteblatt.de/foerderpreise/verleihung?id=2304
Angiosarcoma
Contact
Kontaktinformationen
- Telefon: +49 551 3920643
- E-Mail-Adresse: ramani(at)med.uni-goettingen.de
Cooperations
- Thorsten R. Döppner, Department of Neurology, UMG
- Stefan Nessler, Institute of Neuropathology, UMG
- Ralph Krätzner, Henry G. Klemp und Sven Thoms, Department of Neuropediatrics, UMG
- Bernhard Michalke, Helmholtz Zentrum München
- Hamed Alborzinia, HI-STEM, DKFZ, Heidelberg
- Kostas Pantopoulos, Lady Davis Institute, Department of Medicine, McGill University, Canada
- Ashley I. Bush, The Florey Institute of Neuroscience and Mental Health, Melbourne, Australia
- Michael Aschner, Albert Einstein College of Medicine, New York, United States
- Ashraf Ibrahim, David Geffen School of Medicine UCLA, United States
- Patrizia Agostinis, VIB-KU Leuven Center for Cancer Biology, Leuven, Belgium